Role of Mixed Solvation and Ion Pairing in the Solution Structure of Lithium Ion Battery Electrolytes
Daniel M. Seo, Stefanie Reininger, Mary Kutcher, Kaitlin Redmond, William B. Euler, Brett L. Lucht, J. Phys. Chem. C, 2015, 119, 14038 – 14046
Abstract
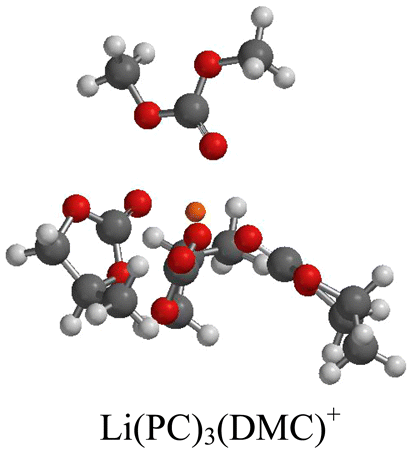
The solution structures of organic carbonate solvents (ethylene carbonate (EC), propylene carbonate (PC), dimethyl carbonate (DMC), and diethyl carbonate (DEC)) as electrolyte solutions of LiPF6 were investigated with FTIR and NMR spectroscopy and DFT computational methods. Both coordinated and uncoordinated solvents are observed by IR spectroscopy, allowing the determination of solvent coordination numbers, which a range from 2 to 5. The predominant species in solution changes as a function of LiPF6 concentration. At low salt concentrations (<1.2 M), the predominant species is a solvent-separated ion pair, whereas at high salt concentrations (>2.0 M) the predominant species in solution is the contact ion pair. In mixed solvent systems (PC−DMC, PC−DEC, EC−DMC, or EC−DEC), the mixed solvated cations are observed in the presence of high concentrations of uncoordinated cyclic carbonate despite the much larger dielectric constant of the cyclic carbonates than dielectric constant of linear carbonate.