Detection of Dinitrotoluene Isomers Using Reactions With Bases: A Structural, Spectroscopic, Kinetic, and Computational Study
Sangmin You, Christopher A. Latendresse, Syrena C. Fernandes, Kathleen M. Sullivan, William B. Euler, Sens. & Actuat. B: Chem., 2015, 216, 165 – 175
Abstract
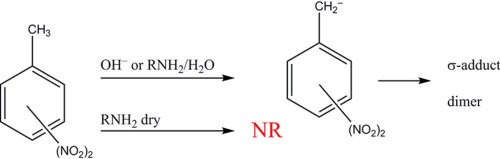
The reactions of dinitrotoluenes (DNT) with hydroxide ion or wet alkylamines can be used to detect and identify each isomer. The reaction products give visible spectra that can be used directly to determine the isomer speciation with sensitivities in the low micromolar range. NMR spectroscopy in dimethylformamide provides unambiguous identification of the products of each DNT when the base is hydroxide. Kinetic studies show that the initial product of this reaction is the deprotonated anion followed by subsequent formation of σ-adducts and, in some cases, a dimer, with the exception of 2,4-DNT, which does not react beyond the acid–base reaction. Alkylamines do not react with any DNT, even when the amine is acting as the solvent, unless there is water present. Water is the limiting reagent in these cases, implying that in all cases the reacting species is the hydroxide ion, not the free amine. Computational studies are consistent with the inability of the alkylamine to deprotonate the methyl group in any of the DNT isomers. A general mechanism that is applicable to hydroxide and amine bases is proposed.