Convenient Syntheses of 2,2'-Biindole
Darrell J. Koza and William B. Euler, Heterocyclic Communications, 1999, 5, 399 – 402
Abstract
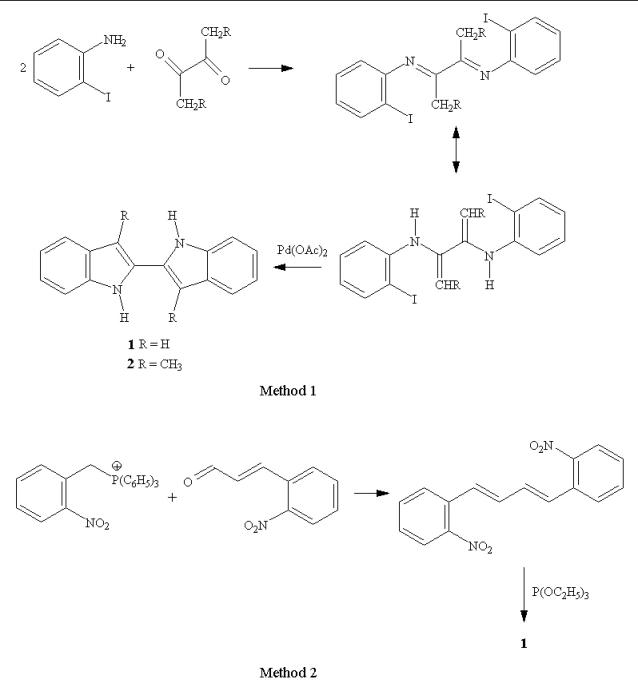
Two new syntheses for 2,2'-biindoles are reported. The first uses a palladium catalyzed annulation of a bis(o-iodophenyl)-α,β-diimine. The method employs mild conditions, gives good yields, and is potentially tolerant of a variety of substituent groups. The second method uses a Wittig reaction between a 2-nitrobenzylphosphonium salt and 2-nitrocinnamaldehyde to form 1,4-bis(2-nitrophenyl)butadiene, which is followed by triethylphosphite cyclization to give the biindole.