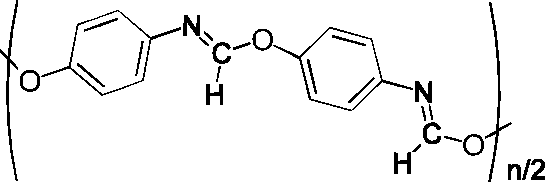
LFER Correlation of 13C Chemical Shift in Para-Substituted Phenyl Isocyanide: Implications for the Formation of a Unique Polymer
Misoo Kim, William B. Euler, and William Rosen, J. Org. Chem., 1997, 62, 3766 – 3769
Abstract
The correlation of Hammett σ constants to the 13C chemical shifts of the isocyano carbon in p-substituted phenylisocyanide examples has been studied. An excellent correlation was found with one exception: p-hydroxyphenylisocyanide. This result implied that this exception has hydrogen bonding between the para-OH group and the isocyano group, which leads to an uncatalyzed polymerization with formation of a unique type of polymer, poly(iminoformate ester).