Catalytic Thermal Decomposition of NO2 by Iron(III) Nitrate Nonahydrate-Doped Poly(Vinylidene Difluoride)
Lasanthi Sumathirathne, Carson Lawrence Hasselbrink, Dugan Hayes, William B. Euler, ACS Omega C, 2022, 7, 43839 – 43846
Abstract
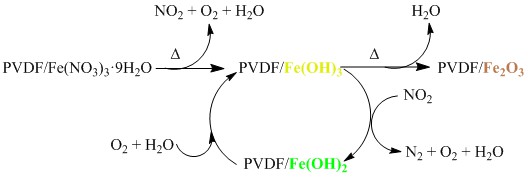
The products of thermal decomposition of iron nitrate nonahydrate doped into poly(vinylidene difluoride) are examined using Mössbauer spectroscopy. Very little of the expected nitrogen dioxide product is observed, which is attributed to Fe3+ catalysis of the decomposition of NO2. The active site of the catalysis is shown to be Fe(OH)3 in the polymer matrix, which is, unexpectedly, reduced to Fe(OH)2. Thermodynamic calculations show that the reduction of Fe3+ is exergonic at sufficiently high temperatures. A reaction sequence, including a catalytic cycle for decomposition of NO2, is proposed that accounts for the observed reaction products. The role of the polymer matrix is proposed to inhibit transport of gas-phase products, which allows them to interact with Fe(OH)3 doped in the polymer.